What is hydrogen?
Hydrogen is a chemical element, 14 times lighter than air, colour-/odourless, non-toxic and does not break down the ozone layer which is essential for human life. With a share of about 75 percent, a large part of the mass of our solar system consists of this gas.
It is a component of almost any organic compounds on earth and is in many respects the perfect fuel and raw material of the future.
As an energy carrier, it can be used in almost all energy-consuming areas – i.e. mobility, heat and electricity.
Therefore, hydrogen plays an indispensable role in the energy revolution. Unfortunately, hydrogen in its molecular form as H2 hardly occurs in nature.
It is mostly bound in water, H2O.
Up to now, sustainable hydrogen has been and is produced by electrolysis, by splitting water using regenerative electrical energy.
SYNTHEC FUELS is taking a new path:
Hydrogen is also bound in the form of hydrocarbons!
For example, in waste wood, waste paper, fermentation residues from biogas plants, liquid manure or in plastic and packaging waste.
Hydrogen, which is literally lying on the road… as waste!
The SYNTHEC FUELS Circular Waste-to-Hydrogen Economy
Use cursor to interact:
Comparison of Hydrogen Production Technologies
By using waste materials as feedstock SYNTHEC FUELS hydrogen is far more economic than Green Hydrogen and it is more sustainable than Gray or Blue Hydrogen.
Green Hydrogen is produced by electrolysis of water, using only electricity from renewable energy sources (Wind and solar energy) for the electrolysis. Regardless of the electrolysis technology chosen, hydrogen is produced CO2-free, as the electricity used is 100% from renewable sources and is therefore CO2-free.
Grey Hydrogen is obtained from fossil fuels. During production, natural gas is usually converted into hydrogen and CO2 under heat (steam reforming).
The CO2 is then released unused into the atmosphere, thereby intensifying the global greenhouse effect: the production of one tonne of grey hydrogen produces around 10 tonnes of CO2.
Blue Hydrogen is grey hydrogen, but its CO2 is captured and stored during its production (carbon capture and storage, CCS). The CO2 produced during hydrogen production does not escape into the atmosphere and hydrogen production can be considered CO2-neutral in the balance sheet.
Turquoise hydrogen is produced by the thermal cracking of methane (methane pyrolysis).
Instead of CO2, solid carbon is produced.
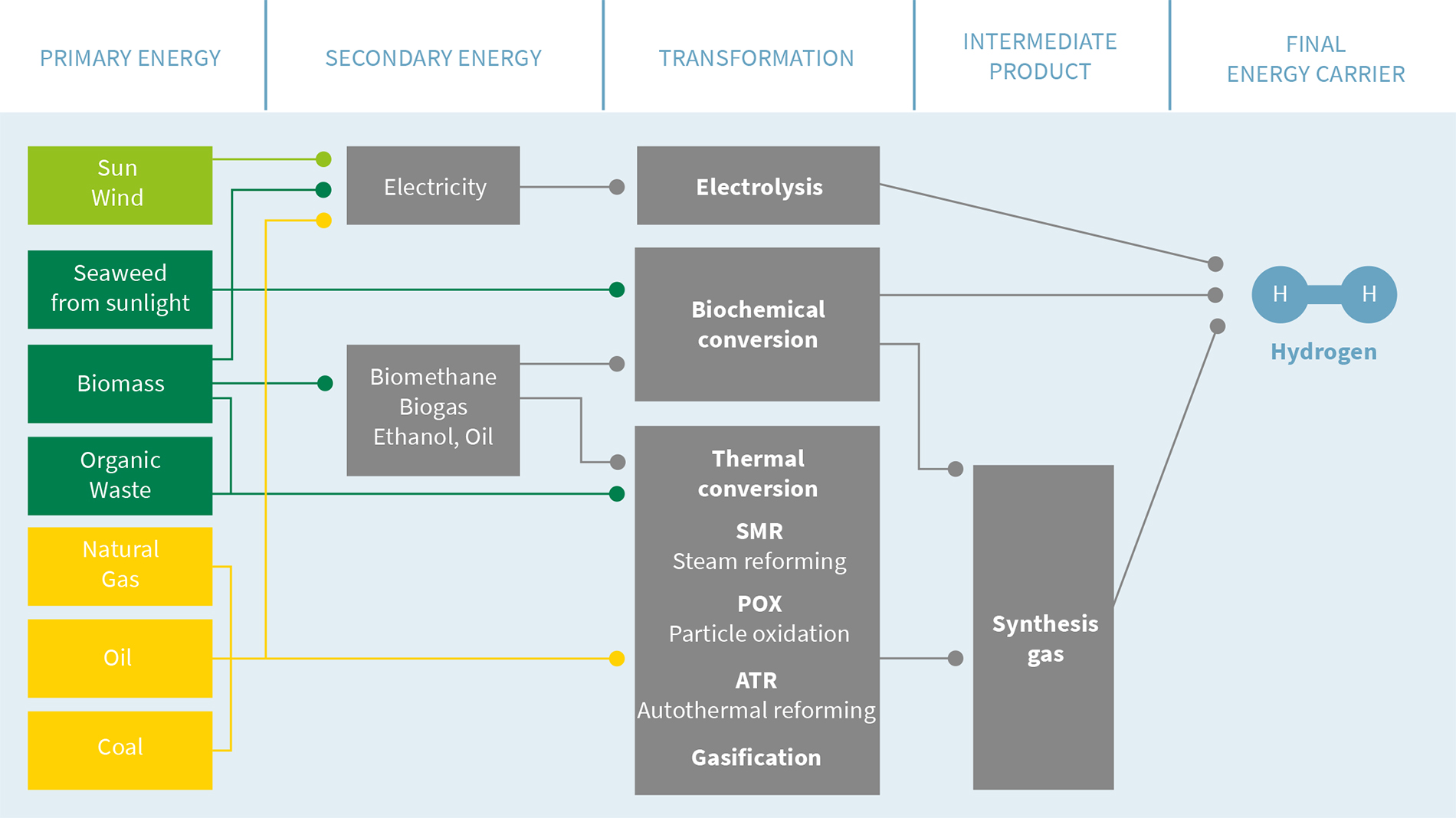
Processes for the production of hydrogen
Costs vs. Energy Density
Comparison of Hydrogen vs. Battery Storage
COSTS
H2-Storage
H2-High-Pressure-Tank
(350 bar)
Batterie-Storage
Li-Ion Battery
(for commercial vehicles)
Hydrogen (H2) in Comaparison to Batteries: 23 x lower Price
GRAVIMETRIC ENERGY DENSITY
H2-Storage
H2-High-Pressure-Tank
(350 bar)
Batterie-Storage
Li-Ion Battery
(for commercial vehicles)
Hydrogen (H2) in Comparison to Batteries: 25 x higher Energy Density
H2-Efficiency and Eco-Balance
Hydrogen has clear Advantages compared to Batteries

With the same storage weight
12 times the range is achieved
With the same storage weight
12 times the range is achieved
With the same range, the entire vehicle is
2-3 times less expensive

Significant advantages in life cycle assessment / „eco-balance“ (For CO2, particulate matter, resources & water)
Hydrogen as “Zero-Emission-Energy-Carrier”
Hydrogen as a Zero-Emission-Energy-Carrier is needed to overcome the challenges of the energy transition

1. Increasing renewables share leading to imbalances of power supply & demand

2. Infrastructure needs to go through a major transformation

3. Global buffering capacity based on mostly fossil sources



4. Some energy uses are hard to electrify via the grid or with batteries:
- Long-range transport
- Energy-intensive industry
- Part of residential heating
5. Carbon needs to be reused to decarbonize feedstock
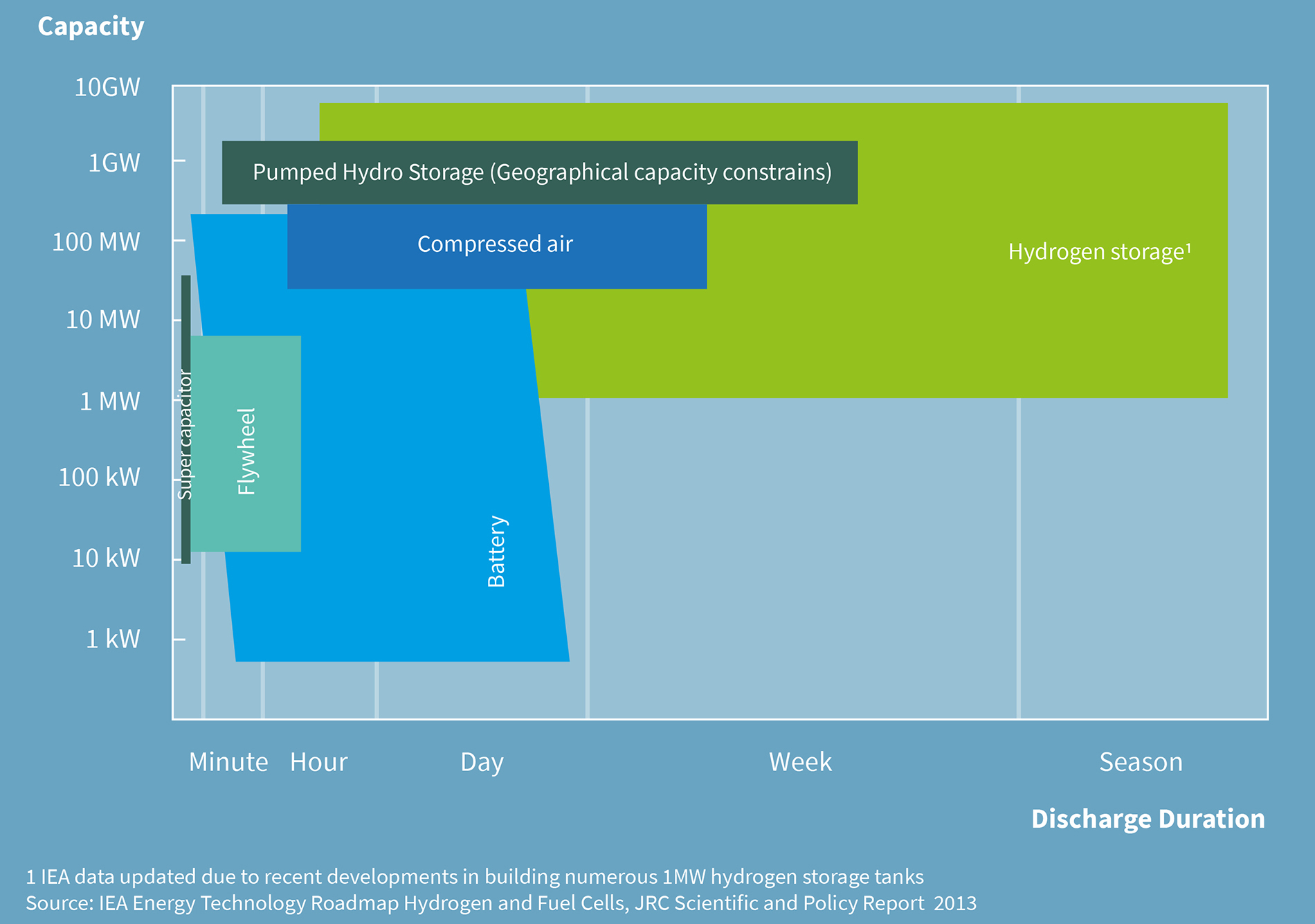
Hydrogen for Long-Term and Carbon-Free Seasonal Storage
Technology Overview of Carbon-Free Energy Storage technologies
Advantages of hydrogen as an energy carrier
In terms of mass, hydrogen has the highest energy content of all fuels.
One kilogram of hydrogen contains about as much energy as three liters of Gasoline or Diesel.
Another advantage of hydrogen is that its “combustion”, i.e. the reaction with oxygen in a Fuel Cell, produces only water. This leads to lower CO2 emissions and no fossil fuels are consumed.
Also read “The Greenhouse Effect”
Advantages of SYNTHEC FUELS Hydrogen
- The energy content of the produced sustainable hydrogen comes from waste which already exists in abundance worldwide and not from surplus high-quality renewable electricity
- Plant availability: 7.500 full load hours per year including two maintenance revisions
- It is far more economic than Green Hydrogen and more sustainable than Gray and Blue Hydrogen
- With hydrogen from waste we can reduce our import demand for oil and gas
- Avoidance of over-fertilisation and contamination of groundwater by recycling liquid manure, sludge such as fermentation residues, manure or sewage sludge in accordance with the EU – Nitrate and Water Framework Directive
- Reduction of waste – landfills including their ongoing maintenance, monitoring, decommissioning and decades of aftercare
- Unnecessary incineration of waste that could be recycled and avoided
- Sensible and valuable reuse of plastic waste
- Connection of the energy sector – waste management – mobility – district development
- By producing hydrogen with the innovative SYNTHEC FUELS technology we optimally fulfill the demands in the German “Kreislaufwirtschaftsgesetz – KrWG” (waste management law)
- Consumption of only 0,2 litres Water per 1 kg sustainable hydrogen produced, compared to a consumption of 9,0 litres drinking water per 1 kg Hydrogen if produced via electrlyzer
9 kg of water for a 1 kg of hydrogen, that is the stoichiometric value. Technically, due to the water treatment usually required, a higher, often even a significantly higher quantity of raw water is needed, especially if seawater is desalinated for this purpose. Taking the average value of the approximately 16,000 desalination plants in operation in 177 countries worldwide, 1 l of fresh water is produced from 2.5 l of raw water (data from: The state of desalination and brine production: A global outlook). If water from these plants were used for hydrogen production by water electrolysis, the technical water requirement would be almost 22.5 kg of raw water per 1 kg of hydrogen.
